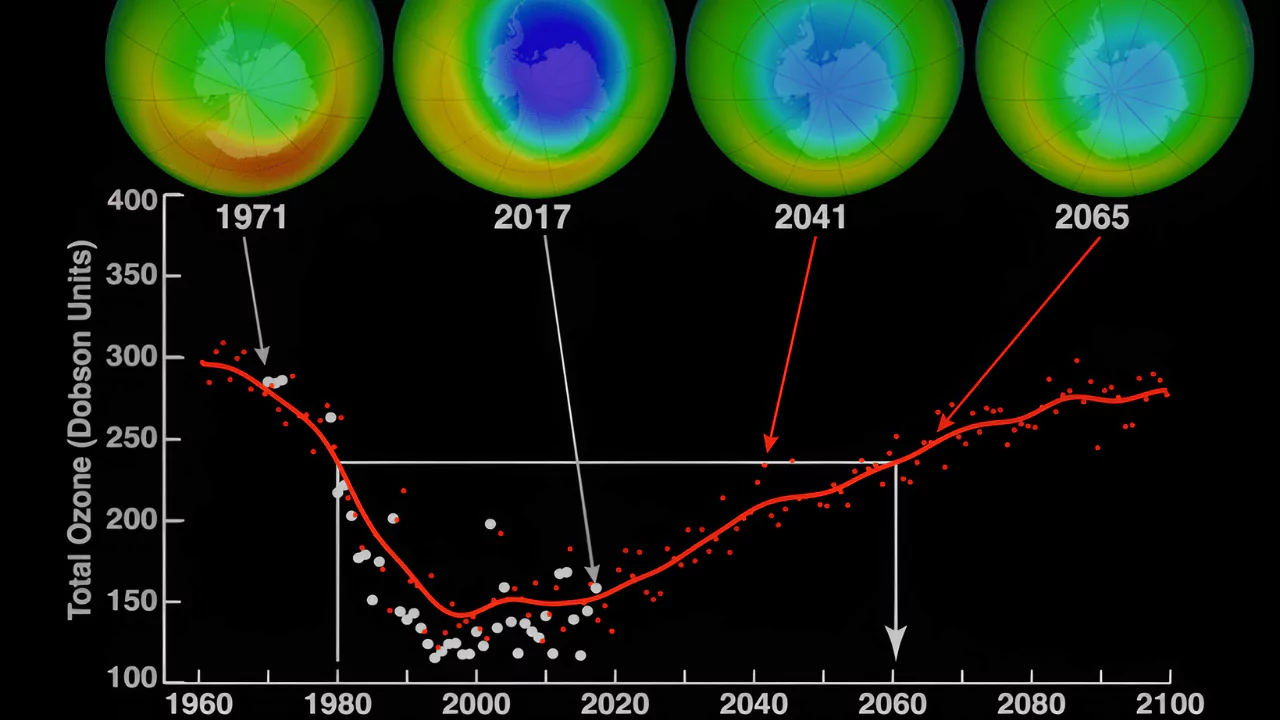
Are you curious about what happened to the ozone hole that once posed a significant threat to our planet? This harmful void in our protective atmospheric blanket was primarily caused by human activity, specifically the use of chlorofluorocarbons (CFCs).
Our article delves into this important environmental issue, explaining its causes, consequences, and importantly, how international intervention helped curb this looming hazard. Let’s set out on a journey to understanding why protecting Earth’s ozone layer is vital for all life forms.
Understanding the Ozone Hole
The ozone hole is a thinning of the ozone layer in the stratosphere, caused by human activities that release harmful chemicals known as chlorofluorocarbons (CFCs).
What is Ozone Layer?
The ozone layer is a crucial part of Earth’s atmosphere, stretching about 10 to 30 kilometers above the planet’s surface. This thin shield contains high levels of ozone (O3), a molecule consisting of three oxygen atoms.
Playing an essential protective role, the ozone layer absorbs most of the sun’s harmful ultraviolet (UV) radiation, which can cause various health problems and environmental damage if it reaches Earth’s surface in large amounts.
The reduction in this protective layer due to the release of chlorofluorocarbons (CFCs) and halons into the atmosphere leads to what scientists call ‘ozone depletion‘, creating an ‘ozone hole’.
What Caused the Ozone Hole?
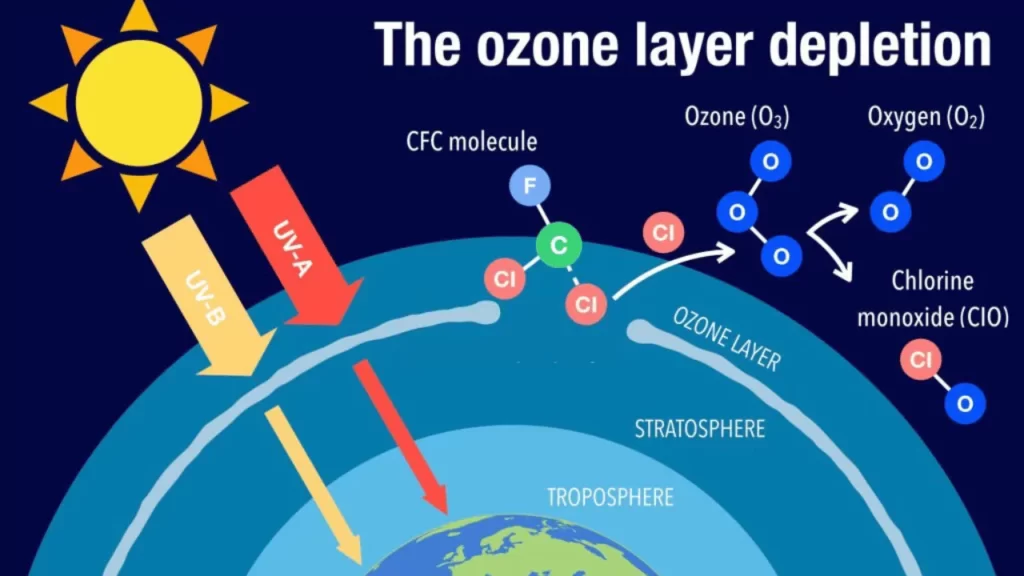
Chlorofluorocarbons (CFCs) and halons are the primary culprits behind the creation of the ozone hole. These chemicals, often found in aerosol spray cans and refrigerants, ascend into the atmosphere after their release.
High atmospheric levels attract these light yet stable compounds where they remain inert until they are broken apart by solar radiation.
Once fragmented by sunlight, CFCs, and halons unleash chlorine and bromine atoms. These atoms become catalysts for ozone destruction—each one can break down thousands of ozone molecules before it is removed from the upper atmosphere.
The breakdown accelerates with the return of sunlight in spring resulting in an annual thinning known as the ‘ozone hole’.
Importance of the Ozone Layer
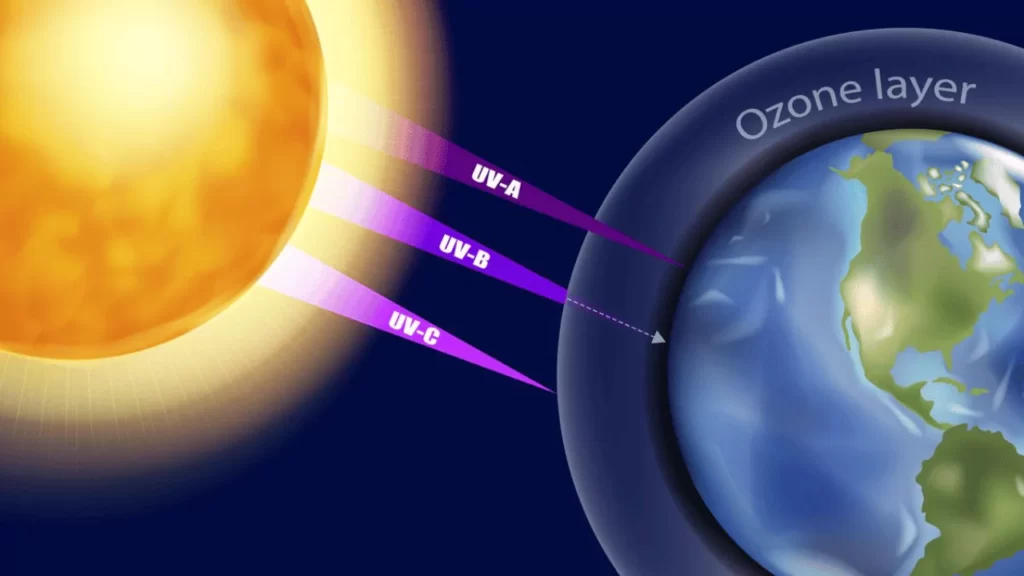
The ozone layer holds immense significance for the survival of life on Earth. Shielding us from harmful UV-B radiation, it acts as a protective barrier against the sun’s most damaging rays.
This absorption of UV radiation by the ozone layer helps maintain our planet’s temperature balance and keeps our environment habitable.
Depletion of this critical layer threatens not just human health but also impacts plant growth and agricultural productivity. Increased exposure to UV-B rays can cause skin cancer in humans and affect plant photosynthesis, disrupting ecosystems at multiple levels.
Protecting this natural shield is crucial to preserving biodiversity and sustaining life as we know it.
Human Activities Leading to Ozone Depletion
Human activities are the main cause of ozone depletion. Some common human activities that contribute to this problem include:
- Emission of halogen source gases: These gases contain chlorine and bromine atoms, which can react with ozone molecules in the atmosphere.
- Use of chlorofluorocarbons (CFCs): CFCs were widely used in aerosol sprays, refrigeration, and air conditioning systems. When released into the atmosphere, they break down and release chlorine atoms that destroy ozone.
- Release of hydrochlorofluorocarbons (HCFCs): HCFCs are commonly used as substitutes for CFCs. Although they have lower ozone depletion potential, they still contribute to the problem.
- Industrial processes: Certain industrial processes release chemicals that can deplete ozone, such as solvents, adhesives, and foam-blowing agents.
- Burning fossil fuels: The burning of fossil fuels releases pollutants into the air, leading to air pollution and contributing to climate change. These pollutants can also indirectly impact the ozone layer.
Effects on Human Health, Plant Growth, and Agriculture
Increased exposure to ultraviolet (UV) radiation, caused by ozone depletion, poses significant risks to human health. This includes an increased incidence of skin cancer and cataracts.
To prevent these health issues, it is crucial to protect the ozone layer. Ozone depletion also has negative effects on plant growth and agriculture. Plants can be damaged, leading to reduced yields in crops and commercial forests.
Additionally, ground-level ozone formed by pollutants can harm vegetation and ecosystems further exacerbating the environmental impact. It is important to address this issue to safeguard our health, preserve plant productivity, and mitigate air pollution and global warming effects.
The Montreal Protocol

The Montreal Protocol is an international agreement that played a crucial role in protecting the ozone layer.
What is the Montreal Protocol?
The Montreal Protocol is a global agreement that was finalized in 1987 with the goal of protecting the stratospheric ozone layer. It was created in response to the depletion of the ozone layer caused by industrial chemicals in the 1970s.
The protocol regulates the production and import of ozone-depleting substances, aiming to reduce their concentration in the atmosphere and safeguard the ozone layer.
How did the Montreal Protocol help to protect the ozone layer?
The Montreal Protocol played a crucial role in safeguarding the ozone layer. This international environmental treaty was created to address the issue of ozone depletion caused by industrial chemicals.
It regulates the production and import of ozone-depleting substances, aiming to phase them out completely and reduce their concentration in the atmosphere. Signed by 197 countries, this protocol has achieved universal ratification and is considered one of the most successful global treaties.
Thanks to its implementation, significant reductions in the size of the ozone hole, particularly over Antarctica, have been observed.
Recovery and Healing of the Ozone Hole
International efforts and policies have led to a reduction of ozone-depleting substances, resulting in the recovery and healing of the ozone hole.
Reduction of Ozone-Depleting Substances
Since 1986, there has been a significant reduction in the consumption of ozone-depleting substances worldwide. This positive change can be attributed to the global agreement known as the Montreal Protocol, which was finalized in 1987.
Its main objective is to protect the ozone layer by phasing out the production and use of these harmful substances. The reduction of ozone-depleting substances plays a crucial role in allowing for the recovery and healing of the ozone hole.
It is an essential step towards mitigating both environmental effects and health risks associated with ozone depletion.
The efforts made to reduce these substances have not gone unnoticed, as they have had a measurable impact on our environment. Scientists remain optimistic that with a continued commitment to reducing these harmful compounds, we are on track for a full recovery of the damaged ozone layer.
International Efforts and Policies
International efforts and policies have played a crucial role in addressing the issue of ozone depletion. Here are some key initiatives and actions taken by countries and organizations around the world:
- The Montreal Protocol: This global agreement, signed in 1987, aims to phase out the production and use of ozone-depleting substances (ODS). It has been ratified by 197 countries and is considered one of the most successful environmental treaties ever implemented.
- National Legislation: Many countries have enacted laws to regulate the production, import, and use of ODS. These regulations ensure compliance with international agreements such as the Montreal Protocol and help to reduce emissions of harmful chemicals.
- Technological Innovations: Governments and industries have invested in research and development to find alternatives to ODS. This has led to the creation of new technologies and products that are more environmentally friendly, such as hydrofluorocarbons (HFCs) which have less impact on the ozone layer.
- Multilateral Cooperation: Countries have worked together through organizations like the United Nations Environment Programme (UNEP) to coordinate efforts, share best practices, and provide technical assistance to developing nations.
- Public Awareness Campaigns: Governments, NGOs, and international organizations have launched campaigns to raise public awareness about the importance of protecting the ozone layer. These campaigns aim to educate individuals about the harmful effects of ODS on human health and ecosystems.
- Monitoring and Research: Scientists continue to monitor changes in the ozone layer using satellites, ground-based instruments, and atmospheric models. This ongoing research helps policymakers make informed decisions regarding international efforts and policies.
Recent Updates on the Ozone Hole
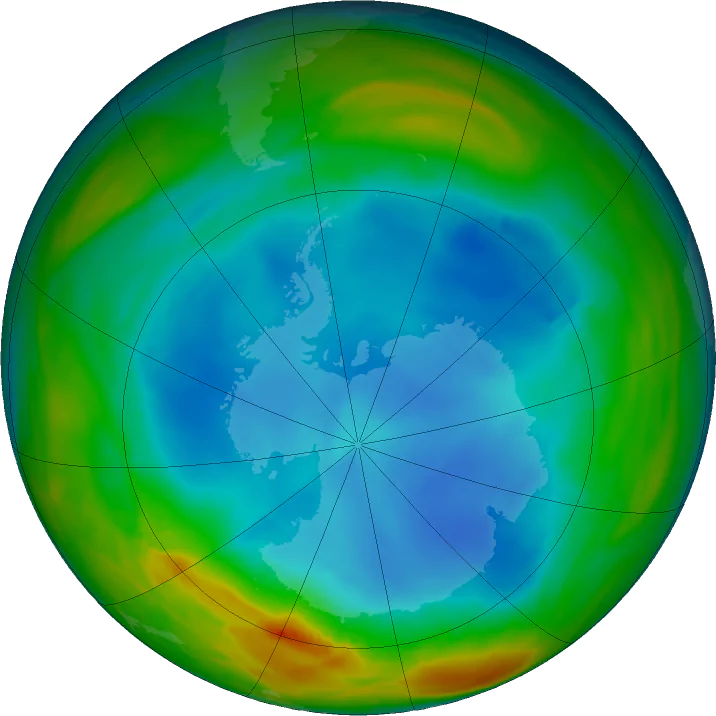
Size and Changes in the Ozone Hole: The ozone hole over Antarctica reached its smallest size in 35 years in 2019, but scientists attribute this to unusual weather patterns rather than a long-term recovery.
Size and Changes in the Ozone Hole
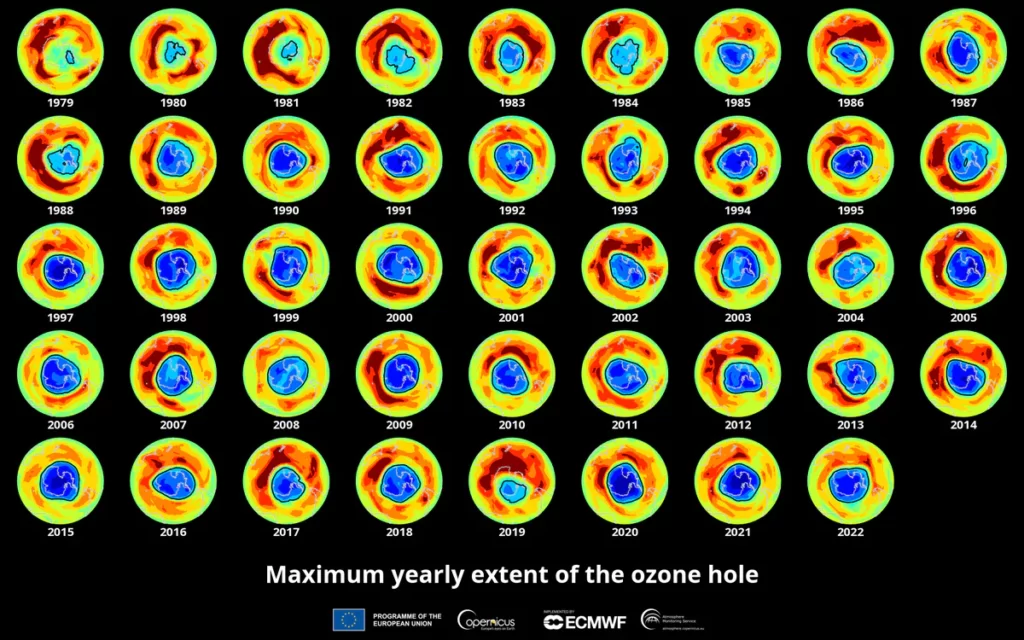
The size of the ozone hole varies from year to year, influenced by factors such as sunlight, temperature, and emissions of ozone-depleting substances. Here is a table detailing the changes in the ozone hole over various years:
| Year | Date | Value |
|---|---|---|
| 1979 | 17 September | 1.1 |
| 1980 | 21 September | 3.3 |
| 1981 | 10 October | 3.1 |
| 1982 | 02 October | 10.8 |
| 1983 | 17 October | 12.2 |
| 1984 | 24 September | 14.7 |
| 1985 | 03 October | 18.8 |
| 1986 | 06 October | 14.4 |
| 1987 | 29 September | 22.5 |
| 1988 | 20 September | 13.8 |
| 1989 | 03 October | 21.7 |
| 1990 | 19 September | 21.1 |
| 1991 | 04 October | 22.6 |
| 1992 | 27 September | 24.9 |
| 1993 | 19 September | 25.8 |
| 1994 | 30 September | 25.2 |
| 1996 | 07 September | 26.9 |
| 1997 | 27 September | 25.1 |
| 1998 | 19 September | 27.9 |
| 1999 | 15 September | 25.8 |
| 2000 | 09 September | 29.9 |
| 2001 | 17 September | 26.5 |
| 2002 | 19 September | 21.9 |
| 2003 | 24 September | 28.4 |
| 2004 | 22 September | 22.8 |
| 2005 | 11 September | 27.2 |
| 2006 | 24 September | 29.6 |
| 2007 | 13 September | 25.2 |
| 2008 | 12 September | 27 |
| 2009 | 17 September | 24.4 |
| 2010 | 25 September | 22.6 |
| 2011 | 12 September | 26.1 |
| 2012 | 22 September | 21.1 |
| 2013 | 16 September | 24 |
| 2014 | 11 September | 24.1 |
| 2015 | 02 October | 28.2 |
| 2016 | 28 September | 23 |
| 2017 | 11 September | 19.6 |
| 2018 | 20 September | 24.8 |
| 2019 | 08 September | 16.4 |
| 2020 | 20 September | 24.8 |
| 2021 | 07 October | 24.8 |
| 2022 | 05 October | 26.5 |
It is important to note that while there are variations in the size of the ozone hole annually, the overall trend shows a reduction due to international efforts to curb the emissions of ozone-depleting substances.
Annual Variations and Natural Factors
Year-to-year fluctuations in the area and depth of the ozone hole are influenced by variations in stratospheric temperature and circulation. Colder conditions in the stratosphere result in a larger area of the ozone hole.
These natural factors play a significant role in determining the size and duration of the ozone hole each year. Additionally, climate change can impact the depletion and recovery of the ozone layer through changes in atmospheric circulation and temperature.
It is important to monitor these annual variations and understand how natural factors interact with human activities to ensure continued efforts to reduce emissions of ozone-depleting substances contribute to healing our atmosphere.
Challenges and Continued Action
The impact of CFCs on ozone layer depletion and the importance of finding alternatives.
Impact of CFCs in Ozone Layer Depletion
CFCs, or chlorofluorocarbons, have had a significant impact on the depletion of the ozone layer. These chemicals are released into the atmosphere through human activities such as industrial processes and the use of aerosol sprays.
Once in the atmosphere, CFCs break down and release chlorine particles that can destroy ozone molecules. This leads to a thinning of the ozone layer, which has serious consequences for our planet.
The depletion of the ozone layer due to CFCs has resulted in increased levels of harmful ultraviolet (UV) radiation reaching the Earth’s surface. UV radiation is known to cause skin cancer, cataracts, and weakened immune systems in humans.
It also affects plant growth by reducing photosynthesis rates and damaging DNA in plants. Additionally, agriculture suffers as UV radiation can lead to reduced crop yields and damage to marine ecosystems.
Efforts have been made globally to reduce CFC emissions through initiatives like the Montreal Protocol. While progress has been made in decreasing CFC production and usage over time, challenges remain in fully recovering the ozone hole.
Alternatives to CFCs
Several alternatives to CFCs have been developed to reduce their impact on the ozone layer and climate change. These alternatives have lower ozone depletion potentials and are considered more environmentally friendly options. Some of the alternatives to CFCs include:
- Hydrochlorofluorocarbons (HCFCs): HCFCs have a lower impact on the ozone layer compared to CFCs. They are still being used as transitional alternatives, but their use is being phased out due to their contribution to climate change.
- Hydrofluoroolefins (HFOs): HFOs are another alternative to CFCs with lower ozone depletion potentials. They have gained popularity in recent years as they have significantly reduced impacts on both the ozone layer and climate change.
- Carbon sequestration: Carbon sequestration technologies help capture carbon dioxide emissions from various sources, including those associated with refrigeration systems that use CFC alternatives. By capturing and storing carbon dioxide, these technologies help mitigate the greenhouse effect and its contribution to climate change.
Potential Threats to Ozone Layer Recovery
Chemical compounds containing chlorine or bromine pose potential threats to the recovery of the ozone layer. These harmful substances, such as chlorofluorocarbons (CFCs), have been widely used in various industries and products.
Even though international efforts, like the Montreal Protocol, have led to a reduction in their production and use, it is important to remain vigilant. The continued emission of these ozone-depleting chemicals can hinder the healing process of the ozone layer and prolong its recovery.
To ensure a successful restoration of the ozone layer, ongoing environmental awareness and action are crucial in promoting sustainable alternatives to CFCs and mitigating their effects on global warming.
Importance of Ongoing Environmental Awareness and Action
Ongoing environmental awareness and action are of paramount importance in addressing the challenges posed by ozone depletion and safeguarding the ozone layer. It is crucial for individuals, communities, and governments to remain vigilant about the impact of human activities on the environment.
By staying informed and actively participating in conservation efforts, we can make a significant difference in protecting our planet.
Continued environmental awareness helps us understand the consequences of ozone depletion, such as increased UV radiation reaching the Earth’s surface and its detrimental effects on human health, plant growth, and agriculture.
Taking action involves promoting sustainable development practices, reducing emissions that contribute to climate change and global warming, controlling pollution levels, preserving biodiversity, utilizing renewable energy sources effectively, and minimizing our carbon footprint.
Conclusion
In conclusion, the ozone hole has been a significant environmental concern with serious implications for human health, plant growth, and agriculture. Thanks to the implementation of the Montreal Protocol and international efforts to reduce ozone-depleting substances, there has been progress in healing and recovering the ozone hole.
However, continued action is needed to address challenges such as CFC alternatives and potential threats to the ozone layer, highlighting the importance of ongoing environmental awareness and action in protecting our planet.