Reader's Favourite
Business
New In Technology

Sponsored
Check Top Writers Review for the best essay writing services
Travel
Featured Reviews
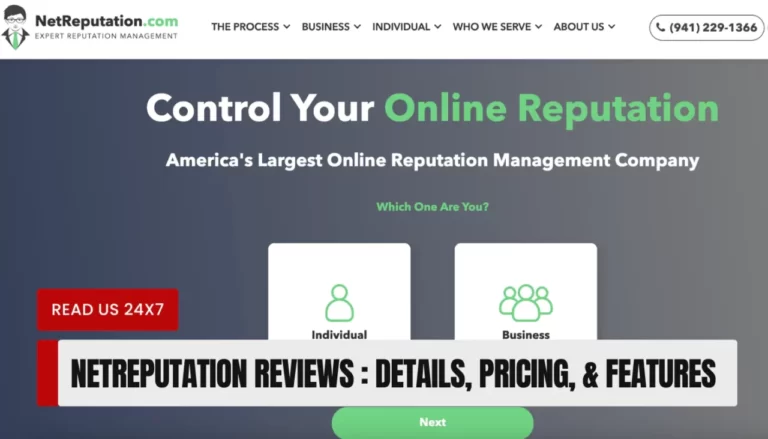
NetReputation is a legitimate and reputable online reputation management service that helps individuals and businesses establish, repair,…
Latest Articles
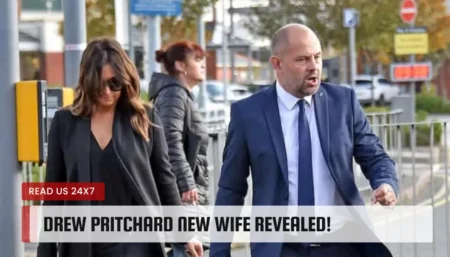
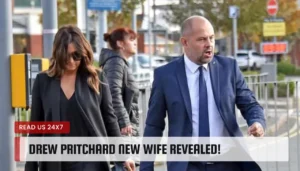
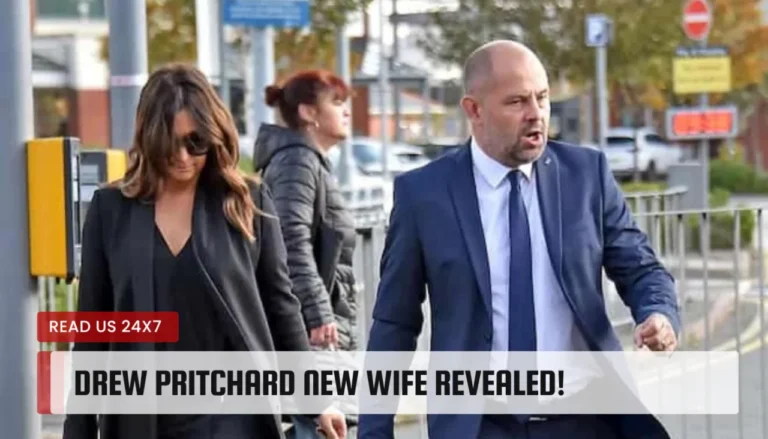
Fans of the show Salvage Hunters were left scratching their heads when co-presenter Rebecca Pritchard disappeared from the show. The charismatic couple,…
The wait is over! After months of speculation and anticipation, the official trailer for “Deadpool and Wolverine” has finally dropped. Fans are…
What is Cubvh? Cubvh is a term that has been popping up in various fields, from design and fashion to technology. While…
For years, backlinks – incoming links from other websites – were considered the golden ticket to high search rankings on Google. But…
Many people love magical stories. “The Little Mermaid” is a story full of magic and adventure. Today, we will explore the mystique…
Have you ever encountered a strange abbreviation or symbol online and wondered what it meant? This is where ftmç comes in. Ftmç…
The New York Times (NYT) crossword puzzle is a beloved daily brain teaser enjoyed by millions worldwide. But for some, the recently…


















![Everything About Project Valvrein [Explained!] Project Valvrein](https://readus247.com/wp-content/uploads/2024/04/project-valvrein-66260c66372d9-450x257.webp)